Description
OBJECTIVE. The aim of this report was to determine the feasibility of fetal cardiotocography during MR imaging and the safety of 1.5-T MR imaging on the basis of fetal heart activity and fetal movements.
CONCLUSION. Fetal cardiotocography is feasible during MR imaging using modified standard equipment. The use of 1.5-T MR imaging appears to be safe and to have no negative short-term effects on the heart rate or movement incidence of healthy third-trimester fetuses under our experimental conditions.
Although sonography remains the principal fetal imaging modality, the advent of ultrafast sequences has increased the indications for fetal MR imaging [1]. At the same time, MR imaging has replaced radiography and CT for pelvimetry and nonobstetric indications in pregnant women because MR imaging avoids exposure to ionizing radiation [2]. To date, no clinical or experimental evidence to our knowledge has indicated that MR imaging may have short- or long-term adverse effects on the human fetus [3, 4, 5, 6]. However, none of these studies tested for fetal distress during the MR imaging examination itself. In the routine clinical setting, fetal welfare is monitored by cardiotocography from week 25 of gestation onward using Doppler sonography. Our aim was to determine both the feasibility of cardiotocography during MR imaging and the safety of 1.5-T MR imaging on the basis of its impact on standard prenatal fetal monitoring parameters.
After our study was approved by the institutional review board, nine pregnant women with uncomplicated singleton pregnancies provided their written informed consent, either at routine prenatal sonography in the obstetric clinic or on referral to the radiology department for clinically indicated MR pelvimetry. All were healthy (mean ± SD: height, 160 ± 4 cm; weight, 74 ± 12 kg; body mass index, 29 ± 5 kg/m2), and prior results of sonography had excluded fetal abnormalities. One patient was excluded from the analysis because of missing cardiotocography data as a result of cable disconnection. In the remaining eight women, the median maternal age was 23 years (range, 18–37 years), and the median gestational age, 36 weeks (range, 33 weeks to 39 weeks 3 days).
Maternal blood pressure was monitored using an extension of the cuff tubing and replacing the metallic parts attached to the cuff with nonferrous material (PressMate BP-8080; Colin, Hayashi, Komaaki-City, Japan). Maternal body temperature was measured from the tympanic membrane outside the 20-mT area with an infrared thermometer (Thermoscan Pro 3000; Braun, Kronenberg, Germany). Because this equipment was used outside the 20-mT area, no modification of the devices was needed.
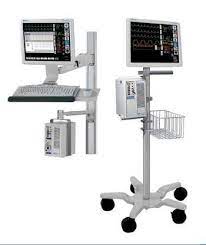
Maternal heart rate was assessed to allow comparison in case of fetal heart rate changes. ECG during MR imaging is already a routine procedure. We perform ECG using commercially available electrodes (RedDot Monitoring Electrode; 3M Health Care, Borken, Germany) connected to an MR imaging–specific high-impedance ECG cable (General Electric Medical Systems, Milwaukee, WI). For this study, the ECG cable output was connected to the cardiotocography device using an in-house 0.25-mm2 copper wire extension lead with polyvinyl chloride insulation and double zinc coating (Fig. 1A).









Reviews
There are no reviews yet.